- Cu Atomic Number
- Atomic Mass Of Cubr2
- 63 29 Cu
- Atomic Mass Of Cu
- Atomic Mass Of Cu2
- Atomic Mass Of Cobalt
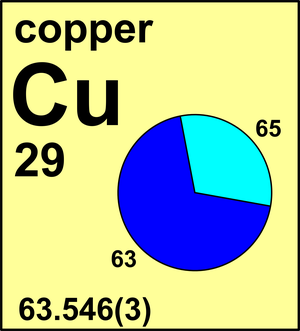
Explanation of how to find the molar mass of CuSO4 - 5H2O (Copper (II) Sulfate Pentahydrate).A few things to consider when finding the molar mass for CuSO4. Estimate The Atomic Mass Of Copper. Copper Has Two Isotopes, Copper-63 (69.15% Abundance) And Copper-65 (30.85% Abundance). (HINT: What Is The Approximate Mass Of Cu-63 In Amu?) 2. An Element Exists As Four Different Isotopes. Refer To The Data Table For The Masses And Abundances. Calculate The Weighted Average Atomic Mass Of This Element. Atomic mass of Copper is 63.546 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. On the periodic table the mass of carbon is reported as This is the average atomic mass of carbon. Carbon atom has a mass of 12.01 amu, but in a handful of C atoms the average mass of the carbon atoms is 12.01 amu. Element Copper (Cu), Group 11, Atomic Number 29, d-block, Mass 63.546. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
Cu Atomic Number
Click to see full answer
Atomic Mass Of Cubr2
Also know, how do you find the percent abundance of copper?63 29 Cu
When it comes to the actual calculation, it's easier to use decimal abundances, which are simply percent abundances divided by 100 . So, you know that copper has two naturally occurring isotopes, copper-63 and copper-65. This means that their respective decimal abundance must add up to give 1 .
Additionally, how do you find the average atomic mass of an isotope? To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together. Whenever we do mass calculations involving elements or compounds (combinations of elements), we always use average atomic masses.
Atomic Mass Of Cu
Keeping this in view, how do you calculate the atomic mass of relative abundance of an isotope?
Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Atomic Mass Of Cu2
What is the percent abundance of an isotope?
Atomic Mass Of Cobalt
The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element.
